Background. Recent studies in genetically high risk (HR) multiple myeloma (MM) such as the UKMRA OPTIMUM study have reported high response rates with intensive induction and post-ASCT consolidation and maintenance strategies, providing the rationale for stratified therapy based on genetic risk. Whilst centralised testing in clinical trials offers ready uniformity and quality control, the use of local laboratories for testing within a clinical trial paves the way for genetic testing to be available for all patients, facilitating risk stratified therapy in routine care. We designed the UKMRA RADAR study to use local laboratories for cytogenetic (CGN) testing to assign treatment according to genetic risk.
Methods. RADAR is an ongoing national, multi-center, risk-adapted, response-guided multi-arm, multi-stage (MAMS) phase II/III trial for patients with newly diagnosed MM eligible for ASCT. Patients receive induction with RCyBorD (lenalidomide, cyclophosphamide, bortezomib, dexamethasone), followed by high-dose melphalan and stem cell rescue. Post ASCT, HR patients are randomised to receive isatuximab or not, alongside RBorD consolidation and R maintenance whilst standard risk (SR) patients receive MRD response guided regimens. A protocol amendment (2022) included isatuximab throughout the HR pathway, removing the post-ASCT randomisation.
Risk assessment, failure rates and turnaround time are overseen by a genomics working group (GWG) including clinical and specialist cytogenetic representation. Genetic risk testing is undertaken in local genetic labs on CD138 selected bone marrow (BM) plasma cells. HR is assigned based on the presence of at least two of: t(4;14), t(14;16), t(14:20), del(17p), del(1p) and gain(1q). Clone size cut-offs are 10% for IgH translocations and 20% for copy number changes. CGN results from local laboratories are centrally reviewed to assign risk in real-time. Indeterminate cases are discussed by the GWG. Repeat testing is mandated if the initial sample fails to yield a definitive risk result for risk. Subjects with no definitive result despite 2 samples are treated as SR.
Results. As of 19 th June 2023, the trial has recruited 472 patients from 72 sites across the UK. Median age is 61 years, 59.3% male, 79.9% white, 11.7% other ethnicity and 8.5% missing ethnicity data. 25 local laboratories were used for CGN testing. Of those with CGN results 354/453 patients (78.1%) were assigned standard risk, 84 (18.5%) high risk and 15 (3.3%) risk category undefined. Risk stratification is ongoing for 19 patients. Of the high risk lesions gain(1q) was most prevalent (35%), followed by t(4;14)(10.6%), del(17p)(8.7%), del(1p32)(8.7%), t(14;16)(3.8%) and t(14;20)(0.8%).
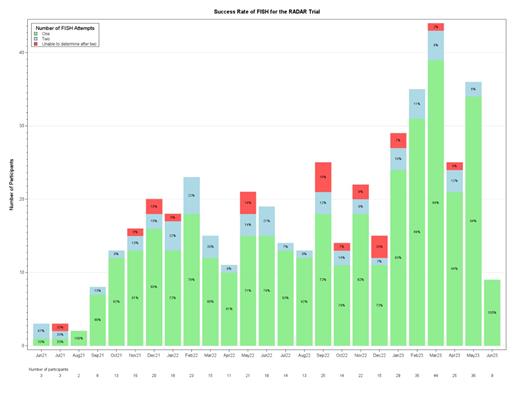
In 376/453 patients (83.0%), risk was assigned from a first BM sample. 77 (17.0%) patients required a second sample; the success rate of repeat samples is 71.4%, 22 (4.9%) patients were unable to be risk-stratified despite 2 samples. Figure 1 shows the success rate of first and repeat tests.
The overall median turnaround time (from D1 cycle 1 until site notified of result) was 11 days (IQR 0 to 21). Sites were sent risk status prior to cycle 1 in 99 (21.9%) patients , 285 (62.9%) prior to cycle 2 and 341 (75.3%) cycle 3. Note this is calculated where treatment data is available.
Conclusion. The RADAR study demonstrates that risk-stratified treatment approaches are feasible in the context of a national phase II/III clinical trial with the use of local laboratories for risk assignment. The vast majority of patients have been successfully allocated to a risk-adapted treatment pathway following a first FISH test (83.0%). Protocol v4 requires time-critical risk-stratification for addition of isatuximab to induction treatment from cycle 2 in high-risk patients, and this has proven to be achievable in almost 2/3 of patients with over ¾ by the end of cycle 2.
The RADAR study also enables hospitals across the UK to access CGN testing via a network model of laboratories with standardization of testing and clinically relevant turnaround times. This model is applicable internationally in both public and privately funded healthcare systems and supports the infrastructure for accessible genetic risk stratification for all newly diagnosed MM patients.
Ongoing studies within the RADAR trial are evaluating novel and clinically deliverable risk stratification protocols to inform risk stratified approaches in newly diagnosed MM.
Disclosures
Kaiser:Karyopharm: Consultancy; Seagen: Consultancy; Takeda: Honoraria; Celgene/BMS: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Regeneron: Consultancy; GSK: Consultancy; Pfizer: Consultancy. Gooding:Bristol Myers Squibb: Research Funding. Cairns:Takeda: Research Funding; Sanofi: Research Funding; Celgene BMS: Honoraria, Research Funding; Janssen: Honoraria; Amgen: Research Funding. Cook:Amgen: Consultancy; Janssen: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Sanofi: Consultancy; Karyopharma: Consultancy. Chapman:Celgene (BMS): Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria. Popat:GSK: Consultancy, Honoraria, Research Funding; Abbvie: Honoraria; BMS: Honoraria; Janssen: Honoraria; Roche: Honoraria. Jackson:Oncopeptides: Consultancy; Sanofi: Consultancy, Honoraria, Speakers Bureau; GSK: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; J&J: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene BMS: Consultancy, Honoraria, Speakers Bureau. Parrish:Gilead: Honoraria; Novartis: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Jazz: Speakers Bureau; Janssen: Speakers Bureau; Everything Genetic: Consultancy; Abbvie: Consultancy, Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; BMS Celgene: Consultancy, Speakers Bureau. Smith:Janssen, Abbvie, Takeda, BMS, Menarini, Sanofi: Honoraria, Other: Support for conference travel and attendance. The research funding referred to above is agreed inprinciple but has not yet been received and may not be until 2024., Patents & Royalties: Honoraria for speaking, assiting in training events, Research Funding. Ramasamy:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotech: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Menarini Stemline: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS ( Celgene): Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Recordati: Honoraria. Jenner:Janssen, BMS, Pfizer, Sanofi: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal